testing hardness of water using titration google scholar|water hardness test method : maker Water hardness from drinking water appears to not be significantly different than tap water. Atomic absorbance is a fast, efficient way to determine water hardness and is an effective .
WEB11 de out. de 2020 · The Mole: Undercover in North Korea: Directed by Mads Brügger. With Ulrich Løvenskjold Larsen, Mads Brügger, Alejandro Cao de Benos de Les y Pérez, Jim Latrache-Qvortrup. A real-life undercover thriller about two ordinary men who embark on an outrageously dangerous ten-year mission to penetrate the world's most secretive .
{plog:ftitle_list}
WEB6.1K members. You like Shemale? Then follow the channel @Sh3mal3Dreams and go to the group to chat in English or German @ChatSh3mal3Dreams.
water test for hardness
why impact test is required for a material
water hardness test method
Titration is one of the most prevalent methods practice in almost all laboratories. Worldwide every curriculum for science and engineering undergraduate students has titrations included in their laboratory sessions. . This paper presents a test method for determining the total hardness in natural and drinking waters using an indicator solution for test titration. The developed method offers .Understanding water hardness. W. Wurts. Published 1989. Environmental Science. Hardness is traditionally measured by chemical titration. The hardness of a water sample is reported in .
Students are taught to calculate the water hardness using their average titration volume by a stepwise calculation involving (1) the amount of EDTA used in the titration, (2) the total . A photometric method is described for the determination in a single titration of both calcium and magnesium hardness in waters using only one indicator.Water hardness from drinking water appears to not be significantly different than tap water. Atomic absorbance is a fast, efficient way to determine water hardness and is an effective .

The developed method offers a rapid and efficient procedure for assessing the total hardness across a wide range of water bodies. The relative standard deviation of a single . A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and .
winchester copper impact test
Hardness of water has traditionally been determined by a complexometric titration with ethylene diaminetetraacetic acid (EDTA). Using the indicator Eriochrome Black .Google Scholar provides a simple way to broadly search for scholarly literature. Search across a wide variety of disciplines and sources: articles, theses, books, abstracts and court opinions. 📏 Method 3: Hard Water Test Strips. Hard water testing strips offer a quick and easy way to test for hard water at home. A DIY water hardness test works by changing color to indicate the minerals present in the water. You .Water hardness is determined by the total concentration of magnesium and calcium. The hardness of a water source has important economic and environmental implications. Currently, titration methods are the most common protocol for the determination of water hardness, but investigation of instrumental techniques can improve efficiency. In this study the hardness of .

The model was applicable for determination of total hardness of water in the range of 116-248 mg L −1 of calcium carbonate. A variety of real water samples were included in the calibration model. The calibration method can be used to predict total hardness of water in a critical range above the soft water and below the very hard water.milligrams of CaCO3 per kg of water. The hardness of water may range from zero to hundreds of ppm, depending on the source. An excellent way to determine hardness of water is to perform a complexometric titration using a standard solution .water, wastewater, surface water, environmental water, raw water. Introduction Total Hardness in water is determined using the preprogrammed method, T7A Total Hard. To determine total hardness, ammonia buffer is added to a sample to adjust pH to 10.0. The sample is then titrated to the equivalence point using ethylenediaminetetraacetic acid
There are 3 steps to determining the concentration of calcium and magnesium ions in hard water using the complexometric titration method with EDTA: Make a standard solution of EDTA. Use the standard EDTA solution to titrate the hard water. Perform calculations to determine the concentration of calcium and magnesium ions in the hard water.
A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies.
There are two main methods of measuring water hardness: titration and test strips. Titration Method. The titration method involves the use of a chemical reagent known as EDTA (ethylene-diamine-tetra-acetic acid) to measure the concentration of calcium and magnesium ions in water. This method is more accurate than using test strips but requires .
In areas of the country where the water is relatively hard, industries might have to spend money to soften their water, since hard water can damage equipment. Hard water can even shorten the life of fabrics and clothes. As the image below shows, long-term movement of hard water through a pipe can result in what is called scale buildup.
Abstract This paper presents a test method for determining the total hardness in natural and drinking waters using an indicator solution for test titration. The developed method offers a rapid and efficient procedure for assessing the total hardness across a wide range of water bodies. The relative standard deviation of a single result in determining the overall . The oxygen saturated water was titrated ten times using 50 mL, V 8, analytical portions where the same Winkler flask was filled two times with water from the lake. This titration was supported on the same blank test and allowed determining the f 2 value (-0.16 mL) and the respective standard uncertainty, u(f 2) (0.15 mL).You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. Both the total hardness and the individual calcium and magnesium hardnesses will be measured. EDTA and the metallochromic indicators used are involved in complexation reactions with the magnesium and calcium ions that are . Determination of water total hardness by complexometric titration general remarks. Water hardness is a measure of the amount of calcium and magnesium salts dissolved in water. There are no health hazards associated with water hardness, however, hard water causes scale, as well as the reduced lathering of soaps. . Depending on the water .
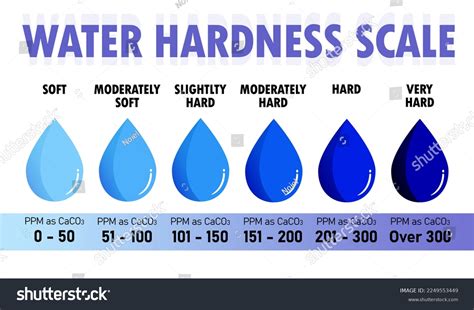
Hardness is traditionally measured by chemical titration. The hardness of a water sample is reported in milligrams per liter (same as parts per million, ppm) as calcium carbonate (mg/l CaCO3). Calcium carbonate hardness is a general term that indicates the total quantity of divalent salts present and does not specifically identify whether calcium, magnesium and/or .
Based on the Mg2 + complexation with acid chrome blue K (ACBK) at pH 10.2, an automatic system was designed to determine total hardness of water. The system consists of a vector colorimeter, a multi-channel sampling pump and both reagents A and B. Two kinds of reagent solutions were prepared and used in this system, i.e., ammoniacal buffer and .DOI: 10.1021/AC50159A028 Corpus ID: 96021776; Determination of total hardness in water employing visual and spectrophotometric titration procedures @article{Fritz1969DeterminationOT, title={Determination of total hardness in water employing visual and spectrophotometric titration procedures}, author={James S. Fritz and John Phillip . In comparison of advanced instrumental techniques, the EDTA titration is simple to operate, can produce accurate results for sample with different complexities, and does not require costly instrumentation. This article focuses on basic principles, titration curves, end point detection, titration types, and practical considerations in EDTA .
commonly used for washing clothes, and when people bathed in tubs instead of using showers, water hardness was more often directly observed than it is now, . 50 mL of the water sample for each titration. As before, add 2 drops of indicator and 5 mL of pH 10 buffer before titrating. Carry out as many titrations as necessary to obtain three . A dip-and-read microfluidic paper-based analytical device (µPAD) was developed for the qualitative and quantitative detection of the total hardness of water. To create well-defined hydrophobic barriers on filter paper, a regular office printer and a commercially available permanent marker pen were utilized as a quick and simple technique with easily accessible .
Titration of calcium and magnesium (total hardness) in bottled and tap water by senior high school students from N. Alikarnassos High School in Crete, Greec.
This is the classic method to determine the total water hardness over a titration with EDTA solution.Patreon: https://www.patreon.com/randomexperimentsintern.
Abstract This paper presents a test method for determining the total hardness in natural and drinking waters using an indicator solution for test titration. The developed method offers a rapid and efficient procedure for assessing the total hardness across a wide range of water bodies. The relative standard deviation of a single result in determining the overall .
This study quantified, classified, analyzed, and compared hardness of tap water samples from household and commercial faucets across the six legislative districts of Manila City, Philippines through complexometric titration. Background: Water hardness varies depending on the source, treatment procedures, and pipeline conditions among others, though not of immediate .Determination of total hardness of water by complexometric titration of calcium and magnesium with EDTA at pH 10 using a Phototrode and Erio T color indicator. Products & Solutions. Laboratory Balances. Industrial Scales. Retail Weighing Scales. Rainin Pipettes and Tips. Analytical Instruments.titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies. The strips contain EDTA and
Total hardness is an important index to evaluate drinking water quality. Total hardness in drinking water was determined by micro titration and conventional titration seperately and parallel determination results of microscale chemical laboratory and constants experiment were compared. The results of micro titration were accurate and reliable. .Estimation of Hardness of Water by EDTA Method 3 In the pH range 8-10, the blue form of the indicator HD2– gives a wine red complex with Mg2+: Mg+2 + HD 2– MgD– + H+ (Blue) (Wine red) Now if EDTA (H2Y 2–) is added to such a solution Mg2+ preferentially complexes with EDTA (since the metal EDTA complex is more stable than the metal-indicator complex) and liberates .
Resultado da 13 de set. de 2016 · @dedaaladimContato profissional: [email protected] gente conversar sobre a vida e afins: .
testing hardness of water using titration google scholar|water hardness test method